1. Model-Based Methods for Cluster Randomized Trials
Cluster randomized trials (CRTs) are studies in which groups—rather than individuals—are randomized to conditions. Clusters may represent hospitals, clinics, and nursing homes, making CRTs indispensable for evaluating interventions delivered at the system, provider, or community level. As healthcare systems increasingly implement complex, multi-component, and policy-driven interventions, CRTs have become central to pragmatic and implementation research. At the same time, they also raise foundational methodological challenges not encountered in individually randomized trials. Addressing such issues is essential for generating credible, interpretable, and decision-relevant evidence in modern clinical and public health research.
The research in my group advances the methodological foundations for designing and analyzing nearly all major classes of cluster randomized trials (CRTs), including parallel‐arm, crossover, and stepped‐wedge designs. This body of work has directly informed national initiatives such as the NIH Pragmatic Trials Collaboratory and the NIA IMPACT Collaboratory, where our methods guide best practices for planning and conducting pragmatic trials. A central methodological challenge in CRTs is appropriately accounting for the correlated outcomes induced by cluster-level randomization to achieve valid, efficient, and interpretable inference. Key areas of my past and ongoing research that adopt a model-based perspective to address clustering include:
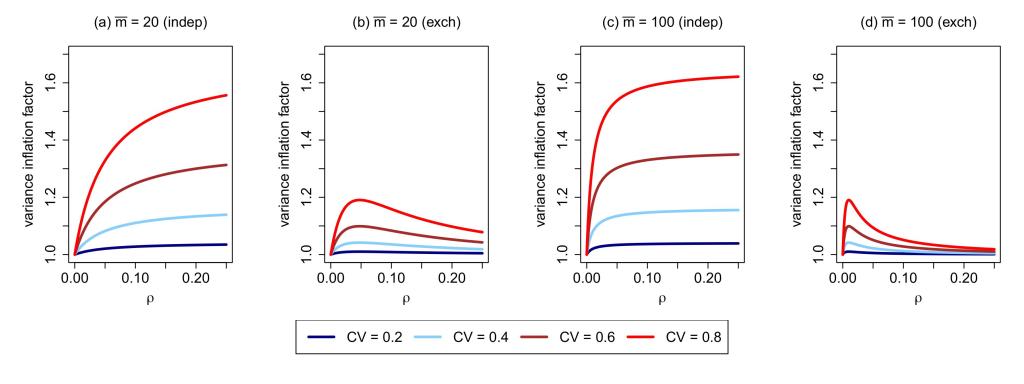
- Covariate-constrained randomization:
- Li F, Lokhnygina Y, Murray DM, Heagerty PJ, DeLong ER. An evaluation of constrained randomization for the design and analysis of group‐randomized trials. Statistics in Medicine. 2016; 35(10):1565-79.
- Li F, Turner EL, Heagerty PJ, Murray DM, Vollmer WM, DeLong ER. An evaluation of constrained randomization for the design and analysis of group‐randomized trials with binary outcomes. Statistics in Medicine. 2017; 36(24):3791-806.
- Zhou Y, Turner EL, Simmons RA, Li F. Constrained randomization and statistical inference for multi‐arm parallel cluster randomized controlled trials. Statistics in Medicine. 2022; 41(10):1862-83.
- Designing parallel-arm CRTs:
- Li F, Tong G. Sample size estimation for modified Poisson analysis of cluster randomized trials with a binary outcome. Statistical Methods in Medical Research. 2021; 30(5):1288-305.
- Wang X, Turner EL, Preisser JS, Li F. Power considerations for generalized estimating equations analyses of four‐level cluster randomized trials. Biometrical Journal. 2022; 64(4):663-80.
- Yang S, Moerbeek M, Taljaard M, Li F. Power analysis for cluster randomized trials with continuous coprimary endpoints. Biometrics. 2023; 79(2):1293-305.
- Designing longitudinal CRTs (e.g., stepped wedge designs):
- Li F, Turner EL, Preisser JS. Sample size determination for GEE analyses of stepped wedge cluster randomized trials. Biometrics. 2018; 74(4):1450-8.
- Davis‐Plourde K, Taljaard M, Li F. Sample size considerations for stepped wedge designs with subclusters. Biometrics. 2023; 79(1):98-112.
- Ryan Baumann M, Esserman D, Taljaard M, Li F. Power calculation for cross-sectional stepped wedge cluster randomized trials with a time-to-event endpoint. Biometrics. 2025; 81(2):ujaf074.
- Designing CRTs with a treatment effect heterogeneity objective: summary of the scientific results here.
- Estimating intracluster correlation coefficients (ICCs):
- Li F, Yu H, Rathouz PJ, Turner EL, Preisser JS. Marginal modeling of cluster-period means and intraclass correlations in stepped wedge designs with binary outcomes. Biostatistics. 2022 Jul 1;23(3):772-88.
- Ouyang Y, Hemming K, Li F, Taljaard M. Estimating intra-cluster correlation coefficients for planning longitudinal cluster randomized trials: a tutorial. International Journal of Epidemiology. 2023 Oct 1;52(5):1634-47.
- Addressing censored survival endpoints in CRTs:
- Blaha O, Esserman D, Li F. Design and analysis of cluster randomized trials with time‐to‐event outcomes under the additive hazards mixed model. Statistics in Medicine. 2022; 41(24):4860-85.
- Wang X, Turner EL, Li F. Improving sandwich variance estimation for marginal Cox analysis of cluster randomized trials. Biometrical Journal. 2023;65(3):2200113.
- Tian X, Ciarleglio M, Cai J, Greene EJ, Esserman D, Li F, Zhao Y. Bayesian semi-parametric inference for clustered recurrent events with zero inflation and a terminal event. Journal of the Royal Statistical Society Series C: Applied Statistics. 2024; 73(3):598-620.
2. Estimand-Aligned Methods for Cluster Randomized Trials
Beyond specifying statistical models, modern analysis of CRTs increasingly demands clarity about “what treatment effect is being estimated“. In CRTs, the presence of unequal cluster sizes, variable treatment exposure, and post-treatment randomization means that model-based analyses may target different causal quantities depending on the choice of working model and scale. This has elevated the role of estimands—well-defined, model-free descriptions of the causal effect of interest—as a cornerstone of contemporary cluster trial interpretation. Estimands are now recognized internationally, including through ICH E9(R1) Addemdum, as essential for ensuring that what is estimated aligns with the scientific question and supports transparent, policy-relevant decision-making.
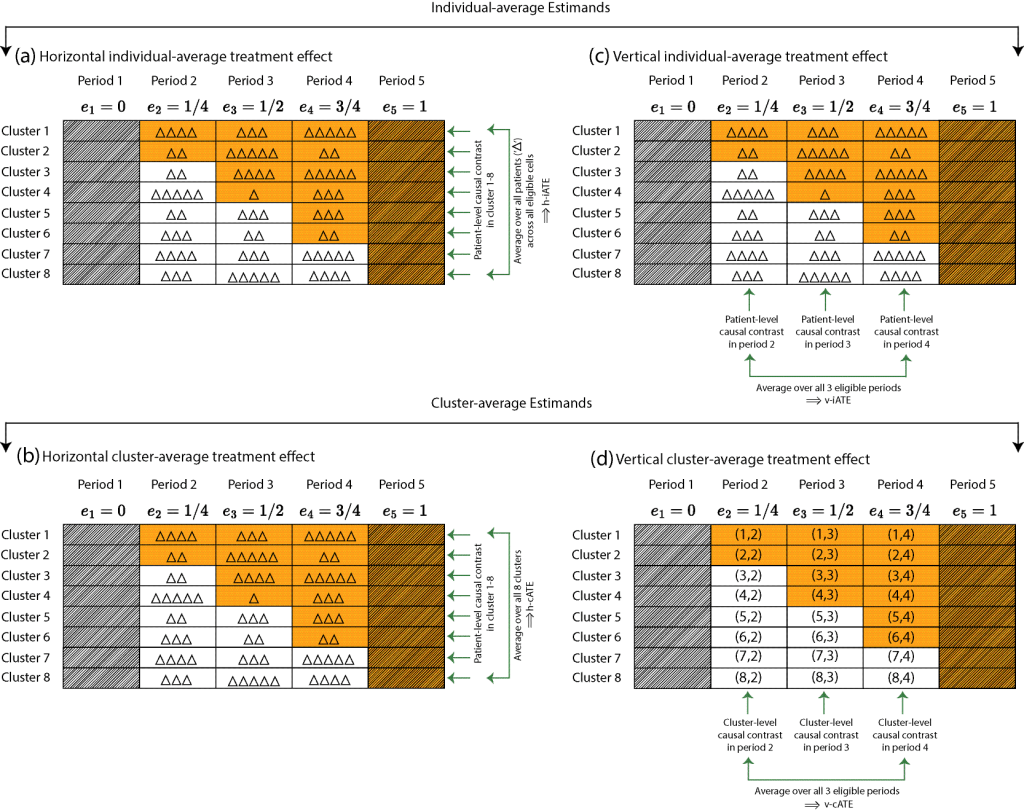
The research in my group contributes to the evolving literature on estimand-aligned frameworks for CRTs that separate the scientific target from the statistical model, clarifying the distinction between cluster-average and individual-average treatment effects and enabling valid inference even under baseline adjustment, informative cluster size and post-randomization bias. Several interest areas include:
- Estimands and model-robust analysis of parallel-arm CRTs:
- Kahan BC, Li F, Copas AJ, Harhay MO. Estimands in cluster-randomized trials: choosing analyses that answer the right question. International Journal of Epidemiology. 2023; 52(1):107-18.
- Kahan BC, Blette BS, Harhay MO, Halpern SD, Jairath V, Copas A, Li F. Demystifying estimands in cluster-randomised trials. Statistical Methods in Medical Research. 2024; 33(7):1211-32.
- Li F, Tong J, Fang X, Cheng C, Kahan BC, Wang B. Model‐robust standardization in cluster‐randomized trials. Statistics in Medicine. 2025; 44(20-22):e70270.
- Estimands and model-robust analysis of longitudinal CRTs:
- Chen X, Li F. Model‐assisted analysis of covariance estimators for stepped wedge cluster randomized experiments. Scandinavian Journal of Statistics. 2025; 52(1):416-46.
- Wang B, Wang X, Li F. How to achieve model-robust inference in stepped wedge trials with model-based methods?. Biometrics. 2024;80(4):ujae123.
- Chen X, Li F. Model-assisted inference for dynamic causal effects in staggered rollout cluster randomized experiments. arXiv preprint arXiv:2502.10939. 2025 Feb 16.
- Fang X, Wang X, Heagerty PJ, Wang B, Li F. Model-robust standardization in stepped wedge designs. arXiv preprint arXiv:2507.17190. 2025 Jul 23.
- Covariate adjustment in CRTs:
- Wang B, Park C, Small DS, Li F. Model-robust and efficient covariate adjustment for cluster-randomized experiments. Journal of the American Statistical Association. 2024; 119(548):2959-71.
- Wang B, Li F. Asymptotic inference with flexible covariate adjustment under rerandomization and stratified rerandomization. arXiv preprint arXiv:2406.02834. 2024 Jun 5.
- Wang B, Harhay MO, Tong J, Small D, Morris T, Li F (2025). On the mixed-model analysis of covariance in cluster-randomized trials. Statistical Science. In Press.
- Addressing selection bias in CRTs:
- Li F, Tian Z, Bobb J, Papadogeorgou G, Li F. Clarifying selection bias in cluster randomized trials. Clinical Trials. 2022; 19(1):33-41.
- Papadogeorgou G, Liu B, Li F, Li F. Addressing selection bias in cluster randomized experiments via weighting. Biometrics. 2025; 81(1):ujaf013.
3. Causal Inference for Addressing Confounding, Moderation and Mediation
Causal inference from observational data is essential for understanding how interventions perform in real-world settings, where treatment assignment is non-random and multiple biases arise. My work develops statistically rigorous methods that enable credible effect estimation in the presence of confounding, measurement error, limited overlap, and complex longitudinal treatment structures. I am interested in advancing estimators grounded in semiparametric efficiency theory, and debiased machine learning to obtain robust average and heterogeneous treatment effects. I also advance causal mediation methods to uncover mechanisms, and develop machine-learning–integrated strategies for effect moderation with valid uncertainty quantification. Some of my example works include:

- Propensity score methods:
- Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. American Journal of Epidemiology. 2019; 188(1):250-7.
- Li F, Li F. Propensity score weighting for causal inference with multiple treatments. The Annals of Applied Statistics. 2019; 13(4):2389-2415,
- Zeng S, Li F, Hu L, Li F. Propensity score weighting analysis of survival outcomes using pseudo-observations. Statistica Sinica. 2023; 33(3):2161-84.
- G-methods for time-varying treatments:
- Hu L, Ji J, Joshi H, Scott ER, Li F. Estimating the causal effects of multiple intermittent treatments with application to COVID-19. Journal of the Royal Statistical Society Series C: Applied Statistics. 2023; 72(5):1162-86.
- Cheng C, Hu L, Li F. Doubly robust estimation and sensitivity analysis for marginal structural quantile models. Biometrics. 2024; 80(2):ujae045.
- Chen X, Hu L, Li F. A flexible Bayesian g-formula for causal survival analyses with time-dependent confounding. Lifetime Data Analysis. 2025; 14:1-28.
- Liu R, Hu L, Perry Wilson F, Warren JL, Li F. A Bayesian approach to the g‐Formula via iterative conditional regression. Statistics in Medicine. 2025; 44(13-14):e70123.
- Causal machine learning for treatment effect heterogeneity:
- Hu L, Ji J, Li F. Estimating heterogeneous survival treatment effect in observational data using machine learning. Statistics in Medicine. 2021; 40(21):4691-713.
- Chen X, Harhay MO, Tong G, Li F. A Bayesian machine learning approach for estimating heterogeneous survivor causal effects: applications to a critical care trial. The Annals of Applied Statistics. 2024; 18(1):350.
- Wang B, Li F, Yu M. Conformal causal inference for cluster randomized trials: Model-robust inference without asymptotic approximations. arXiv preprint arXiv:2401.01977. 2024 Jan 3.
- Maleyeff L, Li F, Haneuse S, Wang R. Permutation tests for detecting treatment effect heterogeneity in cluster randomized trials. Statistical Methods in Medical Research. 2025:09622802251348999.
- Causal mediation methods:
- Cheng C, Li F. Semiparametric causal mediation analysis in cluster-randomized trials for indirect and spillover effects. Biometrics.
- Cheng C, Li F. Inverting estimating equations for causal inference on quantiles. Biometrika. 2025; 112(1):asae058.
- Cheng C, Li F. Identification and multiply robust estimation in causal mediation analysis across principal strata. Journal of the Royal Statistical Society Series B: Statistical Methodology. 2025: qkaf037.
- Ohnishi Y, Li F. A Bayesian nonparametric approach to mediation and spillover effects with multiple mediators in cluster-randomized trials. Journal of the American Statistical Association. 2025; 7:1-13.
- Ohnishi Y, Daniels MJ, Yang L, Li F. Identification and estimation of causal mechanisms in cluster-randomized trials with post-treatment confounding using Bayesian nonparametrics. arXiv preprint arXiv:2510.16673. 2025 Oct 19.
4. Addressing Intercurrent Events under Estimands Framework
Modern clinical research increasingly requires clarity about how treatment effects are defined when intercurrent events, such as death, treatment switching, or non-adherence, interrupt or modify the interpretation of outcomes. The ICH E9(R1) Addendum Estimands Framework has fundamentally reshaped this landscape by emphasizing that analyses must target well-defined estimands that transparently link the scientific question to the treatment effect being estimated. This guidance has made explicit that treatment effects can differ drastically depending on how intercurrent events are handled, and that ambiguity in these choices undermines interpretability, comparability, and regulatory decision-making. A translational article on this point can be found here:
- Kahan BC, Cro S, Li F, Harhay MO. Eliminating ambiguous treatment effects using estimands. American Journal of Epidemiology. 2023; 192(6):987-94.
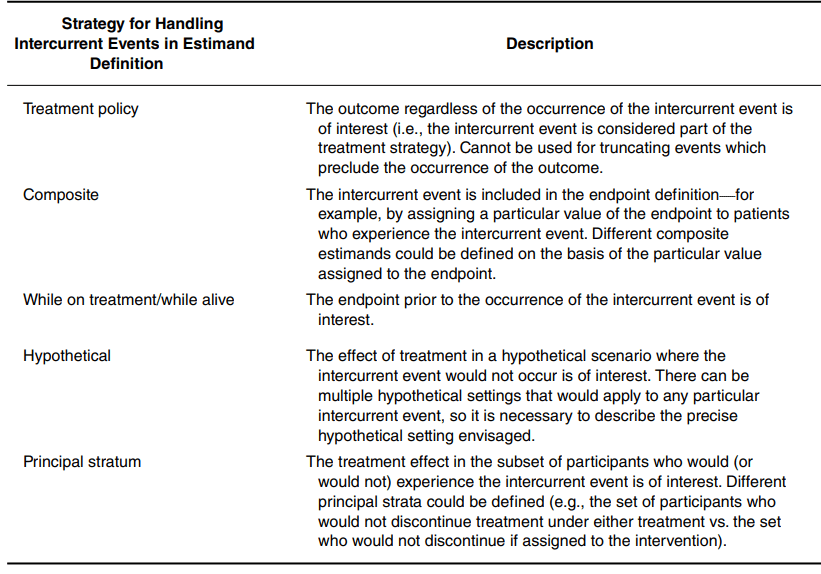
My group advances practice with the Estimands Framework by focusing on three strategies for handling intercurrent events that remain less familiar yet offer substantial room for methodological innovation:
- While-alive strategy:
- Fang X, Uno H, Li F. While-alive regression analysis of composite survival endpoints. Biostatistics. In Press.
- Composite strategy:
- Mou Y, Hummel S, Kyriakides T, Li F, Huang Y. Generalizing the Finkelstein-Schoenfeld test to accommodate multiple alternating thresholds. arXiv preprint arXiv:2407.18341. 2024 Jul 25.
- Cao Z, Zuo S, Baumann MR, Plourde K, Heagerty P, Tong G, Li F. Covariate-adjusted win statistics in randomized clinical trials with ordinal outcomes. arXiv preprint arXiv:2508.20349. 2025 Aug 28.
- Fang X, Cao Z, Li F. Sample size determination for win statistics in cluster-randomized trials. arXiv preprint arXiv:2510.22709. 2025 Oct 26.
- Mou Y, Li F, Esserman D, Huang Y. Correlation matters! Streamlining the sample size procedure with composite time-to-event endpoints. arXiv preprint arXiv:2511.16773. 2025 Nov 20.
- Principal stratum strategy:
- Tong J, Cheng C, Tong G, Harhay MO, Li F. Doubly robust estimation and sensitivity analysis with outcomes truncated by death in multi-arm clinical trials. Statistics in Medicine. 2025; 44(28-30):e70297.
- Cheng C, Guo Y, Liu B, Wruck L, Li F. Multiply robust estimation for causal survival analysis with treatment noncompliance. The Annals of Applied Statistics. 2025. In Press.
- Chen X, Li F. Principal stratification with U-statistics under principal ignorability. Journal of the Royal Statistical Society: Series B (Statistical Methodology). 2026. In Press.
- Tong J, Kahan B, Harhay MO, Li F. Semiparametric principal stratification analysis beyond monotonicity. Statistica Sinica. 2028; 1(38):1-40.
Beyond statistical methodology research, I have a broad interest in translational and collaborative research, including but not limited to cardiology, critical care, geriatrics, nephrology, and implementation science.